- Screening metabolite libraries for their immunosuppressive potential using primary lung/brain cell culture methods
- Chemical optimization of therapeutic leads
- Unravelling their mechanisms of action via RNA sequencing
- Testing efficacy of metabolite regimens to reduce inflammation in mouse models of acute respiratory distress syndrome and multiple sclerosis

The Nencki Institute of Experimental Biology has received a grant from the European Union for the project “New strategies for probiotic supplementation in the prevention and treatment of asthma”.
In recent years, significant progress has been made in understanding the role of microorganisms that inhabit our bodies (also called the microbiome). As a result, there has been a rapid increase in interest in probiotics, which are promoted as “beneficial” bacteria that positively affect various body functions. However, despite marketing claims about the health-promoting properties of probiotics, scientific research shows their low effectiveness in the medical context. There are two key reasons that explain the ineffectiveness of probiotics. The first is the selection of inappropriate bacterial strains, and the second is their lack of effective colonization of the digestive system, which is the main area of microbiome colonization. As part of this research project, we propose new strategies to solve both of these problems. The aim is to investigate the preventive and therapeutic potential of new bacterial strains in the context of asthma and to develop a method to increase the effectiveness of bacterial colonization by opsonizing them with an antibody discovered by us, supporting the development of specific bacterial strains. The approach presented above distinguishes us from existing market solutions, as well as those proposed in patent document databases. Firstly, we propose the use of bacterial strains outside the Lactobacillus and Bifidobacterium families, which dominate the probiotic market. Secondly, we are the first in the world to propose a method to increase the effectiveness of bacterial colonization using a monoclonal antibody. These solutions, based on our innovative and original achievements, pave the way for the commercialization of products that will not only be unique on the market, but above all more effective.
#EU Funds #European Funds

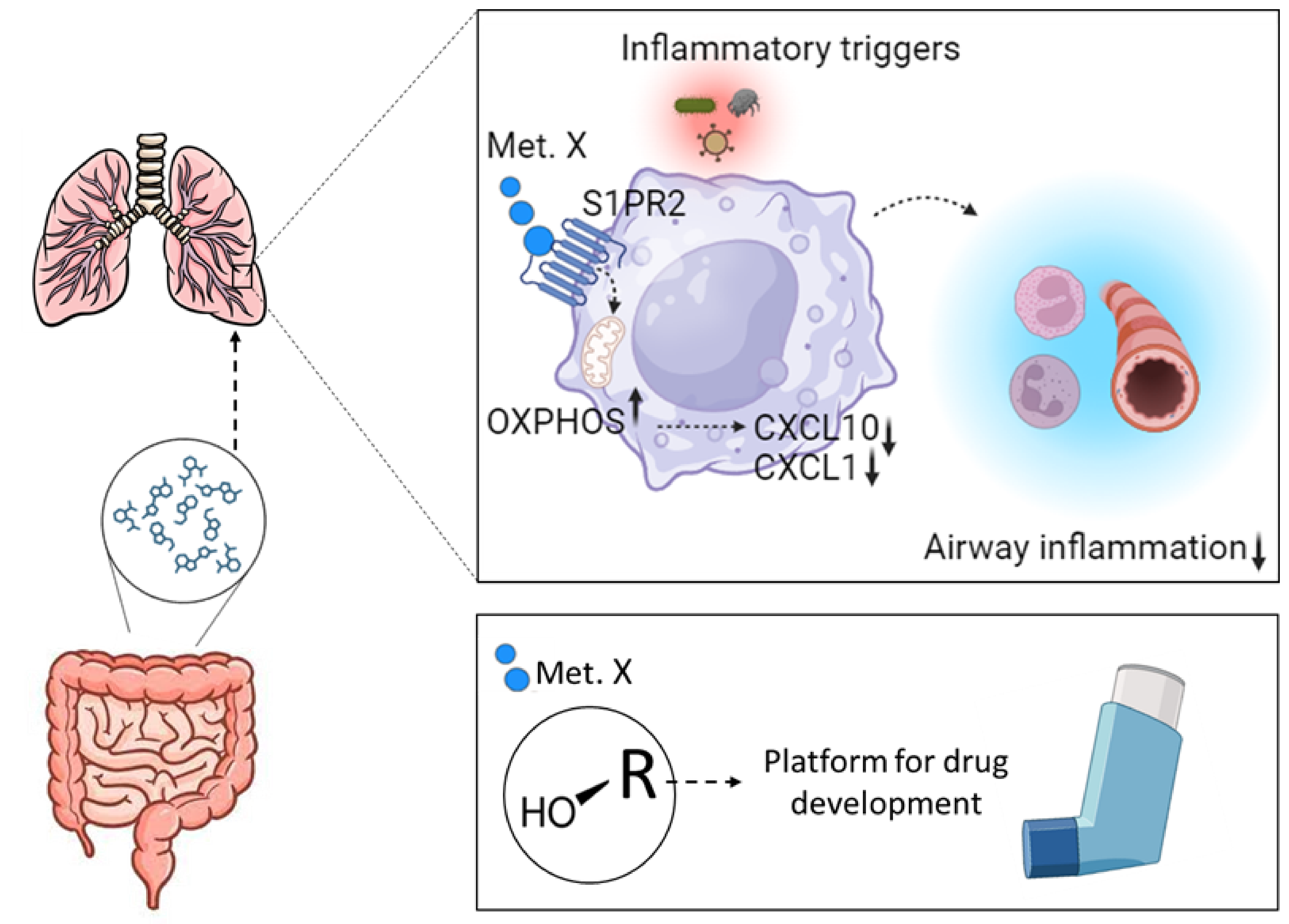
The gut microbiota is a collection of microbes,including bacteria, archaea, fungi, viruses, and protozoa, which inhabit our intestines. It is becoming apparent that the interactions between these microorganisms and human cells are central to maintain health, and become dysregulated in disease.
Despite this, one major question remains unanswered: how does the gut microbiota influence immune function in distal body organs, such as the lungs?
In this project, we are trying to understand this means of communication between the gut and the lungs and identify compounds that can be harnesses therapeutically to ameliorate detrimental immune response in the airways.

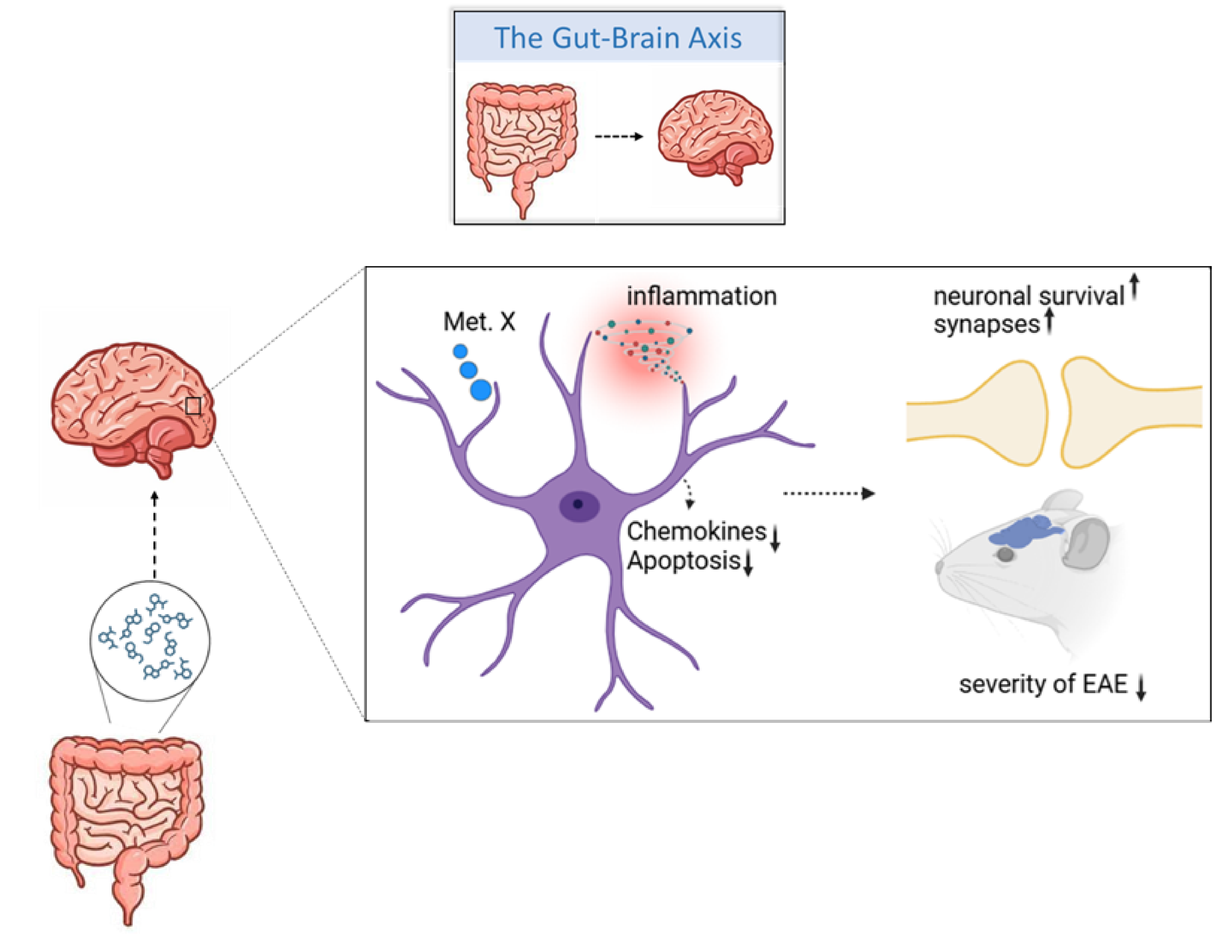
The human body does not exclusively consist of human cells. Instead, it provides an ecological niche to host an equally abundant population of microbial cells, collectively known as the microbiota. An increasing body of evidence points to the fact that these microbes influence the physiology of our organs, including the brain. And although they do not colonize the brain, they can still affect its function. How is it possible?
The key messengers facilitating such a dialogue are small, lipid-soluble metabolites released by the microbes into circulation. Through veins, they can reach the brain, cross the blood-brain barrier and interact with cells. If displaying pro-inflammatory properties, they can contribute to neurodegeneration. If anti-inflammatory, they can prevent it. Unfortunately, we currently know the identity of only a small subset of metabolites active in the brain.
In our preliminary research, we identified a gut-microbiota derived metabolite with profound anti-inflammatory properties in the context of glial cells.This metabolites has never before been described in the context of neurodegeneration and thus, constitutes a potentially untapped source of therapeutic interventions. In this project, we will take the first steps to explore this possibility in the context of multiple sclerosis.
Multiple sclerosis (MS) is a neurodegenerative disease with a staggering mortality rate of 47-75%. In addition, MS patients experience a dramatic reduction in the quality of life caused by physical disability, fatigue, and depression. The microbiota composition in multiple sclerosis is different than in healthy individuals and accumulating evidence points to an enormous potential behind the microbiome-based therapies for MS treatment.
Collectively, in this project we explore innovative approaches to modulate multiple sclerosis outcomes, which might pave the way to translating our research into the clinic.
This project is supported by National Science Centre, grant no. 2021/41/B/NZ6/02219

The tiny living organisms inside our bodies, known as the microbiota, play a crucial role in keeping us healthy. They affect various aspects of our biology, including our immune system. In recent years, we have learntthat these microbes influence the risk of developing asthma.
Because of this, scientists have suggested different strategies to prevent or ease asthma symptoms by targeting the microbiome. These include growing up in rural areas, breastfeeding, eating high-fiber meals, drinking unpasteurized cow's milk, or even being less strict about cleanliness. However, these ideas are often hard to put into practice and might raise safety concerns. So, the key question remains unanswered: can we develop practical and safe solutions to use the microbiome for our health?
Apart from the environment, the microbiome is also shaped by our immune system, which produces specific antibodies. While we used to think that those antibodies only work by eliminating pathogenic bacterial strains, recent evidence shows that it is more complicated: the outcome depends on various factors, and in some cases, these interactions can actually help some bacteria with beneficial properties survive.
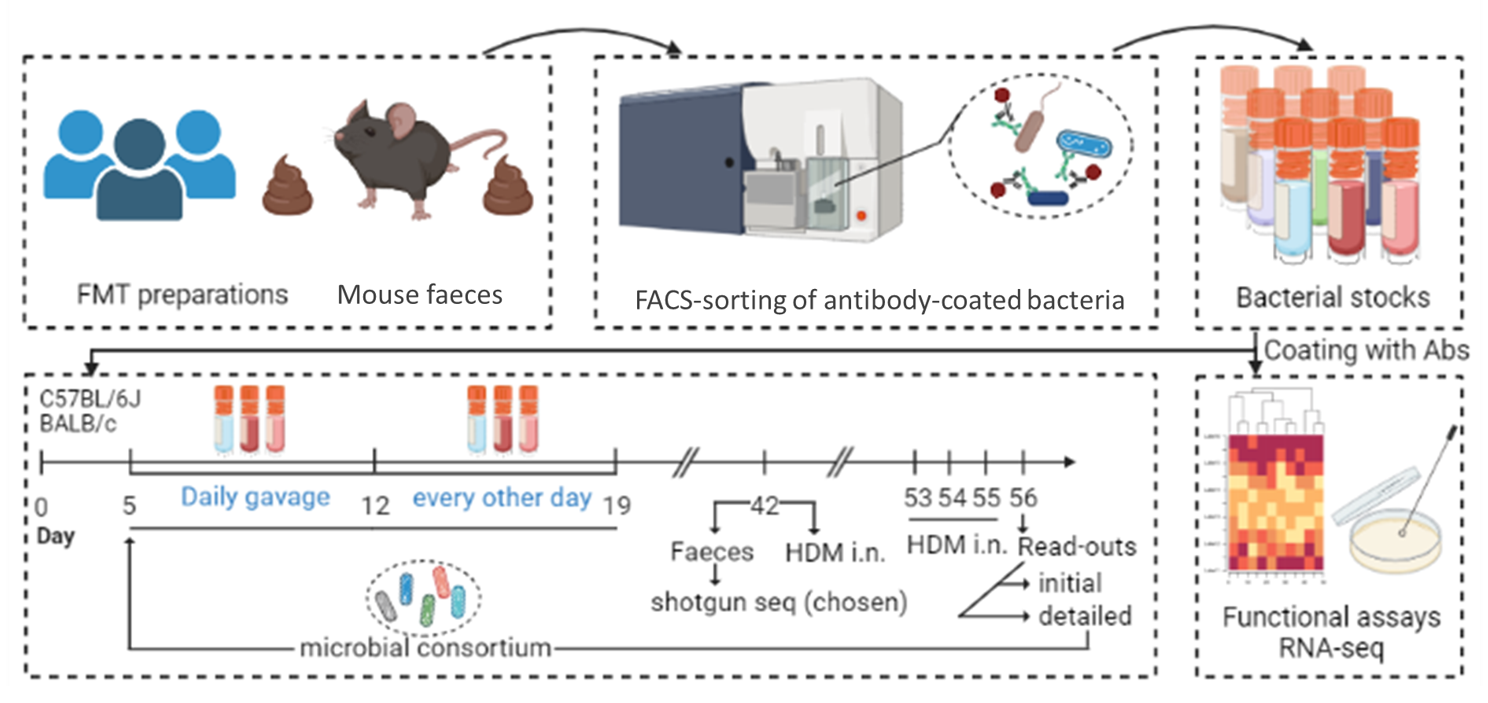
In our previous studies, we discovered a specific antibodyclonethat helps certain bacteria grow, and these bacteria protect mice against asthma.Now, joining efforts with Swiss Institute of Allergy and Asthma Research (SIAF, Dr. Milena Sokolowska),we want to use this information to design vaccines that stably enhance the survival of these bacteria in our gut for the protection against asthma.
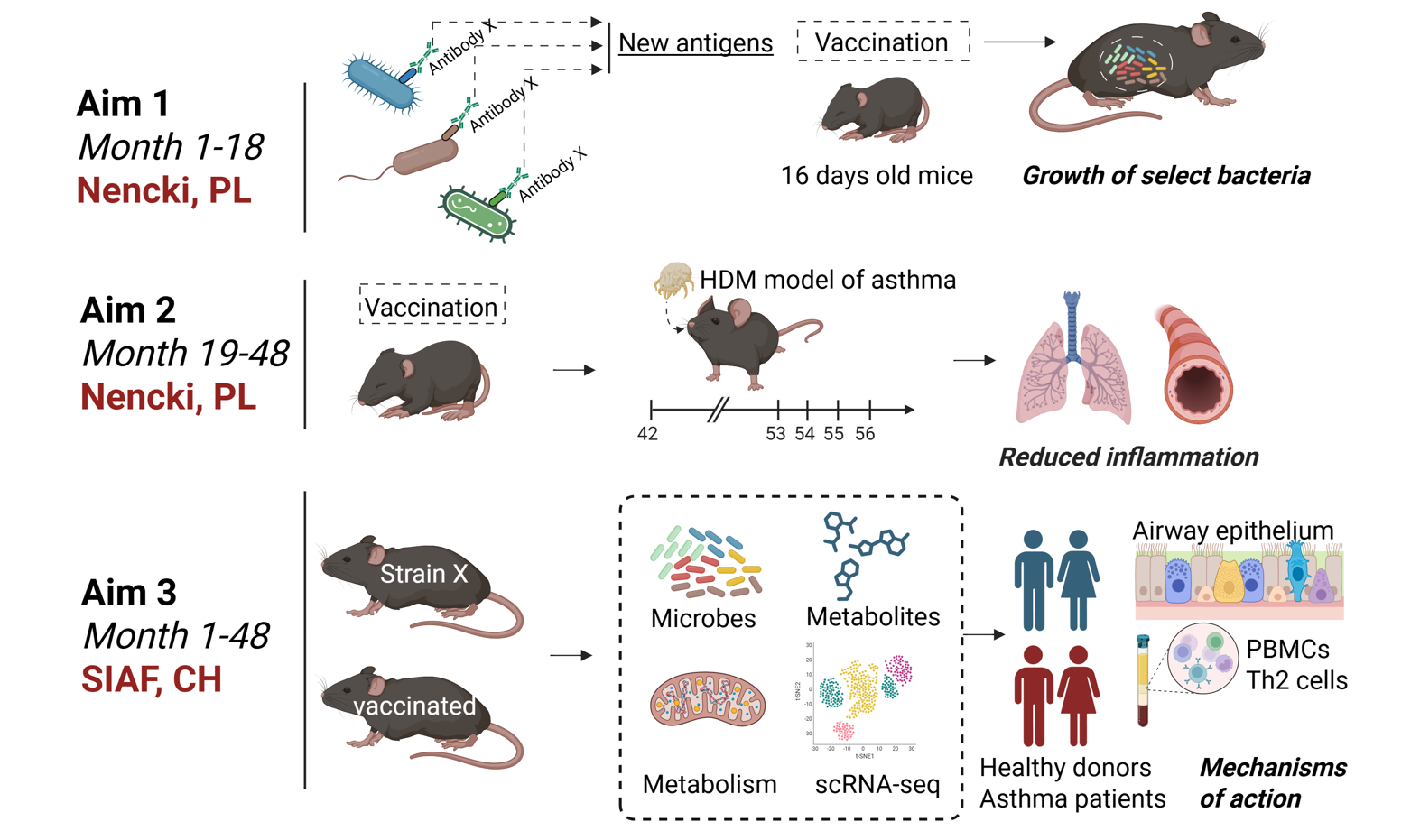
This project is supported by National Science Centre, grant no. 2023/51/I/NZ6/02335

Over the past 15 years, significant progress has been made in understanding the role of microorganisms that inhabit our bodies (also known as the „microbiome”) in shaping physiological processes. As a result, interest in probiotics has surged, with these microorganisms being promoted as „beneficial” bacteria that positively influence various functions of the human body, including our immune system. However, despite marketing claims regarding the effectiveness of probiotics in preventing or treating diseases (including asthma), scientific studies have shown their low efficacy in medical contexts.
There are two key reasons explaining the ineffectiveness of probiotics. The first is the selection of inappropriate bacterial strains, and the second is the lack of effective colonization in the digestive system.
In this research project, we propose strategies to address both of these issues. Our first goal is to investigate the prophylactic and therapeutic potential of novel bacterial strains in the context of asthma. Our second goal is to develop a method to enhance the effectiveness of bacterial colonization (of known and novel probiotics) in the intestine.
These solutions pave the way for the commercialization of products that are not only unique on the market but, most importantly, more effective.
This project is supported by Foundation for Polish Science, grant no. FENG.02.02-IP.05-0106/23
Contact